 Dear BenchFly readers,
Dear BenchFly readers,
I’ve been absent for far, far too long, and before anything else, I’ll need to apologize for that. This new Enzyme Corner article will thus serve a dual-function: 1) telling a new enzyme/protein story, which is far overdue, and 2) bringing you up to speed on what I’ve been up to lately.
.
For those who came in late…
Because it’s been ages since I last wrote for BenchFly, before I tell you what I’m up to now, let me give you a bit of a 5-year summary of where I’ve been and where I am. Five years ago, in June 2008, I was wrapping up my PhD thesis in the Storey Lab at Carleton University in Ottawa, where I studied, in the grand scheme, the molecular mechanisms of cryobiosis; I looked at important metabolic and signaling enzymes in the freeze-tolerant frog, Rana sylvatica, the hibernating Richardson’s ground squirrel, Spermophilus richardsonii, and sometimes poked around with other animals too. At the end of August 2008, I then moved to the Benkovic Lab at Penn State, where I researched for the following 2 years the regulatory controls involved in the reversible assembly of the purine biosynthetic enzyme complex in HeLa cells. Following this, I returned to Montreal, where in August 2010 I began a fellowship known as the Natural Sciences and Engineering Research Council-Industrial Research and Development Fellowship (NSERC-IRDF) at a startup biotech company called Micropharma Ltd. Here, I worked on various aspects of disease-targeted probiotics that were rich in the activity of key metabolic enzymes. I also contributed to the development of an enzyme-driven, nitric oxide-generating medical dressing with antimicrobial and anti-biofilm activity.
Now
Since July 2012, I’ve been at Mount Allison University, the Maclean’s magazine-ranked top primarily-undergraduate university in Canada for 16 out of the past 22 years. I hold the position of the Margaret and Wallace McCain Postdoctoral Fellow, which is actually a bit closer to faculty than the word “postdoctoral” would have you believe. I teach a half-course load, having taught a 4th-year biochemistry course called Immunochemistry last Fall semester, and another 4th-year biochemistry course called Signal Transduction over the Winter semester. For this coming Fall semester, I’m developing a brand-new, never-before-taught 3rd-year biochemistry course called Toxicology.
I also get to participate in research activities, and though the limited-term nature of the 2-year position doesn’t allow me a research infrastructure of my own, I do get to collaborate with faculty who share interests similar to my own. Best of all, as of May, I am now a full-fledged supervisor of my first two very own undergraduate honors thesis students.
As far as research goes, I’m once again back in the domain of comparative biochemistry and physiology, relying on animal models and focusing on metabolic regulation of stress physiology, with a bit of twist: this time around, the stressor is not cold or other naturally-occurring environmental phenomenon, but rather, a man-made toxicant. That toxicant is the spherical nanoparticle.
Nanoparticles
Finally, on to the science! There’s a lot I could say about nanoparticles, but as a biochemist/physiologist, even I’m forced to admit that much of it could be better-said by a materials scientist. For the interests of this article, in a nutshell a nanoparticle can be composed of a variety of elements, both metallic and/or nonmetallic. They are colloidal assemblies of that/those element(s) at the nanoscale- smaller than micrometers but larger than picometers. Because of the way these nanomaterials are assembled, their surface area to volume ratio, and their varieties of surface coatings or functionalizations, they have unique properties; the properties of the colloids are different from the ionic or molecular versions of their constituents (also known as the “bulk” constituents), and also different from the macroscale, solid forms of these materials as well.
Overall, because nanoparticles are roughly on the same scale of a protein’s size, they tend to interact with proteins… and not in a good way. In order to keep nanoparticles in suspension and keep them from agglomerating (or “clumping”) their surface coatings are often charged, allowing them to accumulate bilayers of ions from whatever fluid (biological or otherwise) in which they happen to be suspended (see Figure 1). When they are, indeed, suspended in biological media, the ions they accumulate are not only simple ionized atoms (Na+, K+, Cl–, etc.), but also proteins replete with their own electrostatic surfaces (aspartate, glutamate, lysine, arginine, histidine). A nanoparticle will eventually gather a collection of proteins interacting with its surface, which is becoming more prevalently known in the field of nanotoxicology as a “protein corona.” Proteins can interact reversibly with the nanoparticle surface, associating and dissociating if the interaction is weak enough. Given enough time and the right proteins, however, some proteins may adsorb more strongly to the nanoparticle surface than others, forming a tighter and overall a more irreversible association.
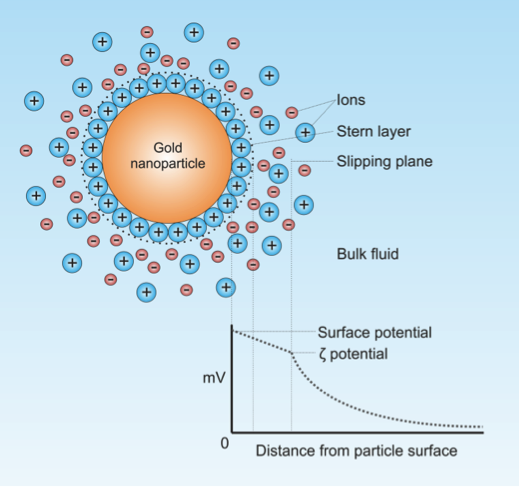
Fig 1. Depiction of a gold nanoparticle and the layers of ions formed around it that allows it to remain in suspension. (Source: Wikipedia, Creative Commons)
The protein
Many scientists in the field of nanoscience study the effects of these protein corona from the perspective of the nanoparticle. How does this change the surface physical and chemical properties of the nanoparticle? How does this affect the disposition (distribution, biotransformation, and excretion) within a living organism? And so on and so forth. However, these questions essentially ignore the co-star of the nanoparticle-protein interaction. In other words, what about the protein?
Well… think about a protein in its native conformation, in its native (and unperturbed) environment. Now… think about that same protein “stuck” (in some way, shape, or form) to a nanoparticle. Through electrostatic interactions, through Van der Waals forces, perhaps to other proteins that are also associated with the nanoparticle like links on a chain, this association is clearly not a natural one, as ionic or other side-chains on the protein are stretched or compacted in their complex interaction with a nanoparticle.
Needless to say, while a protein may not be outright denatured by its interactions with a nanoparticle, it is most definitely not in what we consider to be a “normal,” native conformation.
Structural-__________ relationships
With most types of molecules or biomolecules that we study in the biological sciences, structure has a direct impact on function. And while the primary amino acid sequence doesn’t change, for all intents and purposes conformational changes are structural changes. If a protein is incorporated into a nanoparticle’s protein corona and its conformation is perturbed, you can make a fairly safe bet that its function is similarly perturbed (see Figure 2).
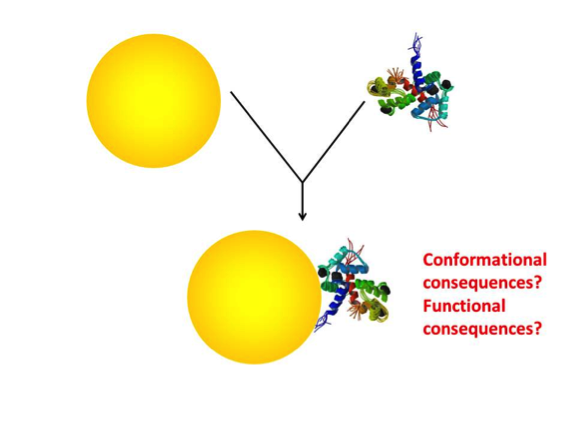
Fig 2. Depiction of a gold nanoparticle and a protein forming an association (the gathering of a “protein corona”) and the pondering of the associated conformational and functional consequences to the protein.
What function, however, are we talking about?
Well, that depends on the protein(s) that is/are interacting with the nanoparticle. Virtually any protein can interact with a nanoparticle, to a weaker or stronger degree, based on the localization of that nanoparticle within an organism. Thus opens the wide field of molecular mechanisms of nanotoxicology.
For instance, are we talking about:
- “Carrier” proteins of otherwise-insoluble ions biomolecules in the plasma?
- Proteins of the innate and adaptive immune systems in the plasma?
- Structural proteins in tissue and cell membranes?
- Catalytic proteins (i.e. enzymes) in mucus, bile, interstitial fluid, plasma or just about anywhere else?
- And then… if a nanoparticle happens to cross the plasma membrane and make its way into the intracellular environment… what happens to membrane, cytosolic, organellar, and nuclear components, protein or otherwise?
Pandora’s box
All the above focused on, for lack of a better term, the physical aspects of the interactions between nanoparticles and proteins. We’ve said nothing so far about the chemical properties of nanoparticles, of which there are many, particularly their generation of reactive oxygen species.
Next time, we’ll look at some more specific mechanistic examples.
 Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa, State College (Penn State), and finally back to Montreal’s biotech industry. Presently, he is the Margaret and Wallace McCain Postdoctoral Fellow at Mount Allison University in Sackville, New Brunswick, Canada. He is researching the role of protein phosphorylation in physiological stress, and teaching 4th-year undergraduate courses in Biochemistry.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa, State College (Penn State), and finally back to Montreal’s biotech industry. Presently, he is the Margaret and Wallace McCain Postdoctoral Fellow at Mount Allison University in Sackville, New Brunswick, Canada. He is researching the role of protein phosphorylation in physiological stress, and teaching 4th-year undergraduate courses in Biochemistry.
What, you missed one of Chris’ previous articles?! Don’t worry, you’re just a click away.
- Make Up Your Mind! When Phosphorylation Turns Enzymes “ON” or “OFF”
- Phosphoproteins: Where’s the “ON” Switch?
- Is There Really Science in the Twitterverse?
- Know Your Role: Enzymes and Their Unexpected Physiological Functions
- Tearing It Up: Glycogen and Its Chemical and Biochemical Breakdown
- Enzymes and the Problem with Cosmo Kramer’s Levels
- What’s in a Name?
.
]]> Previously, I told you about how phosphorylation can turn some enzymes on, and other enzymes off. In my new Enzyme Corner article here on BenchFly, as I’m quite fond of doing I throw yet another monkey wrench into the machine. Today, we discuss how some enzymes can be both activated and inactivated by phosphorylation.
Previously, I told you about how phosphorylation can turn some enzymes on, and other enzymes off. In my new Enzyme Corner article here on BenchFly, as I’m quite fond of doing I throw yet another monkey wrench into the machine. Today, we discuss how some enzymes can be both activated and inactivated by phosphorylation.
Textbook Protein Phosphorylation
When I was an undergraduate in 2nd year biochemistry, one of the first enzymes controlled by protein phosphorylation that I learned about, was the liver isozyme of pyruvate kinase. As the last enzyme of glycolysis, and a step that irreversibly converts phosphoenolpyruvate into pyruvate, it’s a step that needs to be tightly controlled in physiological situations where glucose may be limiting and should preferentially be diverted to other tissues like muscle and brain. When blood glucose is high, the insulin signal is active, which activates/targets protein phosphatase 1 (PP1) to its substrates. PP1 dephosphorylates pyruvate kinase, turning it on. After a time, blood glucose drops. The glucagon or epinephrine signal becomes dominant, which leads to the activation of protein kinase A (PKA). PKA phosphorylates and inactivates pyruvate kinase. Take a look at Fig. 1 for a schematic representation.
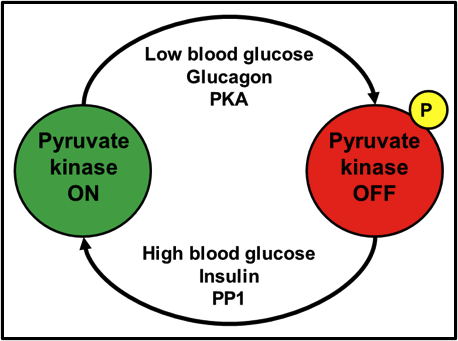
Fig. 1. Activation of pyruvate kinase by dephosphorylation; inactivation by phosphorylation.
This textbook explanation of the phosphorylation-mediated activation/inactivation of an enzyme, albeit accurate, is quite simplistic. For a 2nd year biochemistry student, it really is all they need to know at the time; for those of us who move beyond the 2nd year of our undergraduate studies, however, this type of textbook explanation (and many others across many niches in the biological sciences) engrains within us the perception that an enzyme has one and only one phosphorylation site, and that phosphorylation will either turn an enzyme on or off.
GSK-3: A Servant with Many Masters
Previously, I told you about glycogen synthase kinase-3 (GSK-3), a protein kinase with many substrates- most notably glycogen synthase. However, as is the case with many protein kinases, they have protein kinase kinases of their own that regulate them- such is the way that the hierarchy of signal transduction has evolved.
GSK-3 has two well-known isozymes: GSK-3α and GSK-3β. There is a homologous serine on both these isozymes; Ser9 on GSK-3α and Ser-21 on GSK-3β. Phosphorylation of this serine by an upstream kinase, such as Akt, inactivates GSK-3 and prevents it in turn from phosphorylating its own downstream targets. Interestingly enough, though, there is another site- a tyrosine residue of note. Whereas Akt phosphorylation of Ser9/21 shuts GSK-3 down, phosphorylation of that tyrosine, by protein kinases other than Akt, activates GSK-3 even more.
Ser9/21: Catalytic Site Inhibition
Part of understanding why phosphorylation of one site turns GSK-3 off and phosphorylation another site turns it on (even more), is understanding what each phosphorylation does to the structural-functional relationship of GSK-3.

Fig. 2. A plug chained to a bathtub faucet fits into the bathtub drain. All are connected; essentially, one part of the structure is used to block a hole in another part of the very same structure – Designer Plumbing.
Imagine you’ve had a long day at work and you’re a bath type of person. You turn on the water faucet in your bathtub. If you leave things the way they are, that water is simply going to flow into the drain and downward- you’re going to be waiting a heck of a long time for your tub to fill up, won’t you? So, what do you do? For the sake of proper imagery in this analogy, imagine you have an old-school bathtub with a long chain that connects your faucet to a plug. You put the plug into your drain. Now that the plug is exactly where the water needs to go, it can’t get in! Effectively, by using a plug that was chain-linked to the faucet, you used a piece of bathtub’s own structure to plug a cavity into which something (the water) would naturally fit (Fig. 2).
You can likely see where this is going: Ser9/21 is part of the GSK-3 structure that has the flexibility and potential to curve back inward, all the way to the catalytic core. Since the catalytic core uses ATP to place a phosphate onto a serine or a threonine, a phosphoserine precisely blocks any potential substrate to be phosphorylated from entering the catalytic core, shutting GSK-3 down. Take a look at Fig. 3.
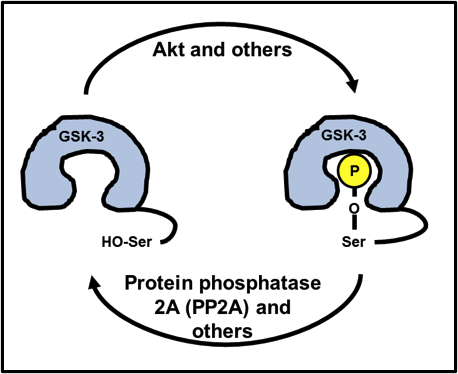
Fig. 3. Inhibition of GSK-3 by Ser9/21 phosphorylation. A part of GSK-3’s own structure folds back into its catalytic core, blocking substrate phosphorylation.
Open Arms (no, not the Journey song): Tyr279/216
Where Ser9/21 phosphorylation inactivates GSK-3, phosphorylation of Tyr279 in GSK-3α or Tyr216 in GSK-3β, activates it. Admittedly, after a thorough literature search, the Tyr-mediated activation of GSK-3 is seemingly less studied and less clear than the Ser-mediated inactivation. I liken Tyr279/216 to a relationship with a loved one. When you need a hug from them and reach in, chances are that a loved one will never push you away (if they do, that’s another issue entirely!), and they’ll hug you back. What makes that hug so much sweeter, though, is when their arms are open and they’re inviting you in for a hug, even before you reach out to them. Tyr279/216 phosphorylation rotates said tyrosine outward, away from the substrate-binding region of the catalytic core, opening up the substrate-binding region even more. Essentially, a phospho-Tyr279/216 GSK-3 is inviting its substrates in for a great big bear hug (Fig. 4)!
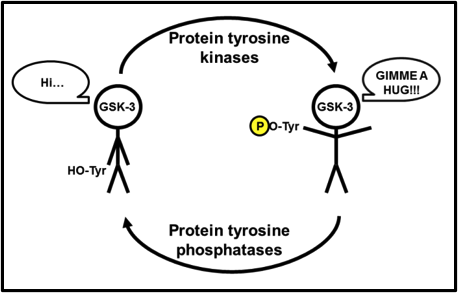
Fig. 4. Further activation of GSK-3. GSK-3 is active even without Tyr279/216 phosphorylation, but tyrosine phosphorylation opens up the substrate binding region of the catalytic core, allowing for greater substrate access.
But… who wins?
What if both Ser9/21 and Tyr279/216 are phosphorylated at the same time? Who wins out: the bathtub plug or the bear hug? Sadly, no matter how much a GSK-3 invites a substrate in for a hug, if the serine or threonine of said substrate can’t reach the catalytic core to be phosphorylated, because phospho-Ser9/21 is blocking the way, the biochemistry of protein phosphorylation simply can’t proceed. Therefore, Ser9/21 inactivation wins out over Tyr279/216 activation. Fig. 5 illustrates the relationships.
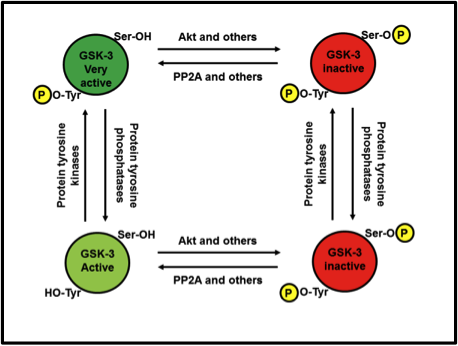
Fig. 5. Relationship between Ser9/21 phosphorylation and Tyr279/216 activation. Tyrosine phosphorylation can turn an active GSK-3 into a very active GSK-3. However, regardless of whether tyrosine is or isn’t phosphorylated, as soon as serine is phosphorylated, it’s game over.
Further reading
While admittedly a dozen years out of date, very few present-day reviews can hold a candle to this review article: Cohen, P. (2000) The regulation of protein function by multisite phosphorylation – a 25 year update. Trends Biochemical Sci. 25: 596-601. Written by the venerable guru of protein phosphorylation, Sir Philip Cohen at the University of Dundee, this piece deals specifically with proteins that are regulated by multiple phosphorylation sites. Highly recommended for more advanced reading!
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
Have any kinase-related questions for Chris?
.
]]>
 Light switches on the wall: Concepts we take for granted
Light switches on the wall: Concepts we take for granted
For much of my life, dealing with electrical lighting has been relatively straight-forward. Upon walking into a dark room, I flip a wall-mounted switch into the up position to turn the lights on. When I’m done in that room, moving that same switch into the down position turns the lights off. For the first 26 years of my life, turning something on or off had essentially become a “dogma” for me: up for on, down for off.
When I began working as a postdoc at Penn State, I moved into an apartment that had a few unique quirks. One of those was that a lot of the light switches moved in a horizontal direction (left or right) as opposed to almost all vertical switches that I had encountered in my life until then (up or down). Suddenly, “up” no longer necessarily meant “on”, and “down” no longer necessarily meant “off.” The dogma that I had memorized for 26 years prior had been broken!
Phosphoproteins: the light switch is always up-and-down… right? Wrong!
One of the questions I’ve encountered many times in my scientific career- usually coming from students- is how protein phosphorylation affects enzyme activity. Is phosphorylation or dephosphorylation what activates an enzyme? Or, in other words, is the “on” form of an enzyme the phosphorylated or unphosphorylated form, and is the opposite the “off” form? When someone asks me this, student or otherwise, I smile, take a deep breath (mostly to prepare for the onslaught of follow-up questions), and say that it depends entirely on the protein being phosphorylated. Moreover, it can even depend on the phosphorylation site, too; a phosphorylation of a serine residue over here can turn an enzyme on, whereas phosphorylation of a threonine right over there will turn the very same enzyme off. Just like that light switch, which I once took as being “dogma,” sometimes there is no universal answer that is guaranteed to cover every situation.
Mixing and matching: Pathways where phosphorylation is both activating and inactivating
I recently worked on a study which was particularly interesting in terms of phosphorylation activating or inactivating enzymes. This particular signalling pathway contained several protein kinases, some of which were activated by phosphorylation, and others which were inactivated, leading down to the final metabolic target of glycogen synthase (GS). Oh, and, insert shameless plug for my recent publication here.
Most scientists who have taken a biochemistry or molecular/cellular physiology course at the 4th year level have at least a passing familiarity with the insulin signalling pathway. Insulin causes the insulin receptor to phosphorylate and activate the insulin receptor substrate-1 (IRS1). IRS1 interacts with and activates phosphoinositide 3-kinases (PI3Ks), which phosphorylate phosphatidylinositol phosphates (PIPs; for example, converting PIP2 to PIP3). PIP3 recruits both phosphoinositide-dependent kinase-1 (PDK1) and Akt to the plasma membrane, allowing PDK1 to phosphorylate and activate Akt. The take home message, for the scope of this article, is that all of the aforementioned systematic phosphorylations- insulin receptor through Akt, inclusive- are activating. I’d draw you a diagram but when it comes to something so widespread like insulin signalling, I can’t compete with the commercial websites, like this one.
What happens after Akt gets to be even more interesting.
Opposite side of the same coin
Akt is turned on when it is phosphorylated by PDK1. However, Akt is now going to use the same trick- phosphorylation- to turn something off…
Only one protein kinase remains in the signalling pathway originating at the insulin receptor and ending with GS: glycogen synthase kinase-3 (GSK3). However, unlike all the kinases described thus far, GSK3 is actually inactivated by Akt phosphorylation; phosphorylation turns GSK3 OFF. Once inactivated, GSK3 cannot, in turn, phosphorylate its own downstream targets. That means that GS remains unphosphorylated. Like GSK-3, an unphosphorylated GS is an active GS; a phosphorylated GS is an inactive GS.
With some phosphorylations being activating and others being inactivating, while writing the manuscript for that publication I shamelessly plugged, even I admittedly had to keep a few post-it notes glued to my wall so that I could remember which phosphorylation patterns led to an activation or inactivation of the final metabolic target. For your benefit, dear readers, in short, here’s what can happen:
1. Akt active, GSK3 inactive, GS active
When Akt has been phosphorylated and activated by its upstream signalling pathway, it phosphorylates and inactivates GSK3. A phosphorylated GSK3 is inactivated. An inactivated GSK3 cannot phosphorylate GS, and therefore GS remains active. Take a look at Fig 1.
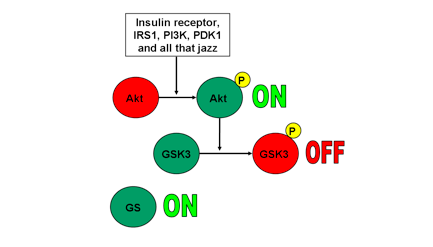
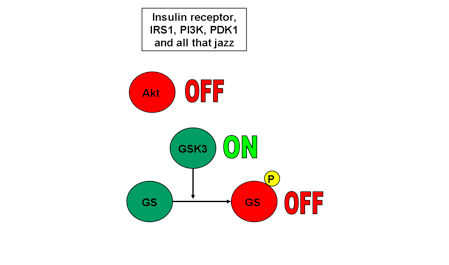
2. Akt inactive, GSK3 active, GS inactive
Now assume Akt has not been phosphorylated by its upstream signalling pathway. An unphosphorylated Akt is inactive. It cannot phosphorylate its downstream targets. Unphosphorylated GSK3 remains active. An active GSK3 phosphorylates GS, inactivating it. Check out Fig. 2.
So the next time a student asks me “Chris, does phosphorylation turn an enzyme on or off?” I’ll refer them to this article. And now, you can do the same if a student asks you!
… oh, and go read my paper… please. Dieni, C.A., Bouffard, M.C., and Storey, K.B. (2012) Glycogen synthase kinase-3: cryoprotection and glycogen metabolism in the freeze-tolerant wood frog. J. Exp. Biol. 215: 543-551.
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
Have any kinase-related questions for Chris?
.
]]> Hello BenchFly readers! It’s been quite awhile since my last edition, and I apologize for my absence. The life of a scientist… you know? But enough of that- down to my latest instalment. I was all set to give you an instructive, fun article on signal transduction, but events over the past few weeks have inspired me to deviate from the standard Enzyme Corner recipe to bring you something different. Without further ado, through the magic of social media I am here to talk to you about… the magic of social media… or is it the myth? In particular, I’m curious about the place of science within the Twitterverse.
Hello BenchFly readers! It’s been quite awhile since my last edition, and I apologize for my absence. The life of a scientist… you know? But enough of that- down to my latest instalment. I was all set to give you an instructive, fun article on signal transduction, but events over the past few weeks have inspired me to deviate from the standard Enzyme Corner recipe to bring you something different. Without further ado, through the magic of social media I am here to talk to you about… the magic of social media… or is it the myth? In particular, I’m curious about the place of science within the Twitterverse.
“A bird can roost but on one branch, a mouse can drink not more than its fill from a river.” – Chinese proverb
I can’t quite remember when I first started using Twitter. In the beginning, it was a big deal; I completely bought into the social media hype. But I also found there were substantial benefits to it. If nothing else, I got introduced to BenchFly through Twitter, and hey we can all relate to that! Truly though, I felt as though Twitter was fast becoming a viable tool that could be used to exchange or present information to a widespread audience- assuming it has not become that tool already. For my part, I’ve tried to contribute to science via Twitter; I always tweet whenever I have a new manuscript accepted (particularly once it’s in electronic or paper print). I tweet when I have a new blog post out. I tweet when I’ve discovered or noticed something interesting about science that I don’t usually see in my everyday routine. I enjoy using Twitter as a means of disseminating information- provided I don’t give away research secrets or divulge something that’s about to go into a publication.
In addition to putting a lot out into Twitter, every so often I try to see if I can maybe get something back. As far as I can tell, there are quite a few scientific experts out there. Certainly, there are the people who run BenchFly and everyone who follows BenchFly! In all seriousness, regardless of whether they follow BenchFly, there are professors, grad students, postdocs, research associates, and lab managers. There are technical and sales reps for companies that manufacture and/or distribute equipment and consumables used for scientific research. There are science writers and science media consultants. Effectively, there is a substantial concentration of science intellect out there in the Twitterverse- in theory a lot of hefty brains to pick.
.
“A bird doesn’t sing because it has an answer, it sings because it has a song.” – Maya Angelou
Like many researchers out there, my research requires me to do things that I have not, as of yet, gained extensive familiarity or expertise with. Or, to put it bluntly, sometimes I’ll run an experiment, or use a piece of equipment or biochemical reagent, and I don’t have the faintest idea of what I’m doing. And so, here in 2012 in the internet age, I can take a little walk from the lab bench to my desk, log onto my Twitter account, and throw out a question or two to the Twitterverse. For instance:
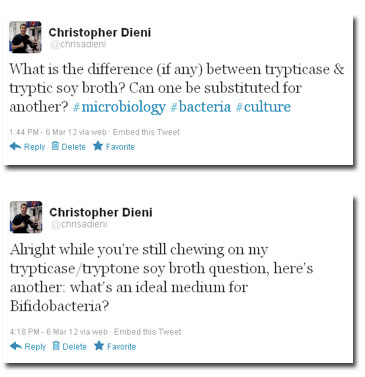
(Yes, I know I typed “tryptONE soy broth” instead of “tryptIC soy broth.” What can I say- the exhaustion of a scientific researcher.)
For those of you who are about to complain that my questions are too microbiology-specific, hold the phone for just one second- here’s something a bit less microbiological:

Are people even reading these tweets? Do I have a substantial presence on Twitter? I’m not sure how to answer that. I can throw out a few numbers, for the curious folk. At the moment of writing this, I have 233 followers. I can confidently say that at least 160 of them have some attachment to science or scientific research. I have also been added to 35 lists on Twitter (do people still use those or have they fallen out of common usage?), 26 of which follow scientists. On top of that, I know for a fact that at least one of the questions above was retweeted by at least 2 people; everyone who follows those retweeters, in turn, should have also seen my tweet. Overall, who knows how many people read my question.
I bet you’re all wondering, what sorts of answers did I get for the questions I tweeted above? How many people inundated me with answers, echoing out across the Twitterverse? Well, I’ll tell you:
Zero.
That’s right, zero. Not a single reply to any of those three questions above (along with many other questions I’ve tweeted in my history on Twitter). Some questions or comments have received replies, over my history on Twitter. Many others, however, have not.
.
“Hold fast to dreams for if dreams die, life is a broken winged bird that cannot fly.” – Langston Hughes
So, what went wrong? What happened to my unshakable faith in Twitter as a viable tool for communications, the exchange of ideas and information? Was it misplaced?
Were my questions too specific for one area (well, no, because from above you can see how it ranges from microbiology to general chemistry)? Was it that, simply, no one had an answer? Was no one paying attention?
.
“I would rather learn from one bird how to sing than to teach 10,000 stars how not to dance.” – E. E. Cummings
Some of you might find this shocking: yes, I do have a life outside of science. Back on Sunday night, for instance, I was watching the season 2 finale of The Walking Dead. With the revelation of Michonne and her katana, and that final camera pan away from the campsite and the view of that prison in the distance, Twitter was on fire with tweets about The Walking Dead! Trending topics on Twitter, during that hour, included “Michonne” and “Lori.” I suppose it was too much of a science nerd fantasy to hope, in parallel, that the comparison of trypticase and tryptic soy broth could spark a similar reaction across social media…?
Perhaps it’s just a case that the science following on Twitter can’t quite compare to that of zombies who eat the living. It raises a question though: if we can’t discuss science- and I’m talking about the nitty gritty minutia of science, down to trypticase and EDTA- on Twitter, then where? The discussion groups on LinkedIn? Facebook? More traditional discussion forums on biology-type websites? But then, even if these types of questions are answered in those old school- oops, I mean traditional- discussion forums, you still need to look for the right website, the right discussion “thread” or category among all the discussion forums, post your question, and hope for an answer (and keep an eye on it, if there’s no automated email alert set up for replies). Why is it that we’re not yet at a point where you can simply log on to Twitter, throw a question out into the Twitterverse and get an answer (or answers)?
Now to you, dear readers: what is your experience, if any, with science and Twitter?
.
Note: There is a poll embedded within this post, please visit the site to participate in this post's poll..
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
Do you think Twitter is a useful or overrated tool for scientists?
.
.
]]> Did you ever see the episode “Mirror, Mirror” in the original Star Trek series? In a nutshell: Kirk, McCoy, Scotty and Uhura get sent- via one of many transporter accidents- to a parallel universe where people and places are the same, but their history and behaviour are strikingly different. Instead of the enlightened Federation, there’s the barbaric Terran Empire its subjugated alien species. Rather than earning promotions, crew members of the ISS Enterprise advance by assassinating their superiors. And in the boldest fashion statement of all, Spock is rocking a beard. The so-called mirror universe is explored again in several episodes of Deep Space Nine. In the first DS9 mirror-themed episode, Kira provides one of the simplest and most elegant explanations of the mirror universe: “the players are the same but everyone seems to be playing different parts.”
Did you ever see the episode “Mirror, Mirror” in the original Star Trek series? In a nutshell: Kirk, McCoy, Scotty and Uhura get sent- via one of many transporter accidents- to a parallel universe where people and places are the same, but their history and behaviour are strikingly different. Instead of the enlightened Federation, there’s the barbaric Terran Empire its subjugated alien species. Rather than earning promotions, crew members of the ISS Enterprise advance by assassinating their superiors. And in the boldest fashion statement of all, Spock is rocking a beard. The so-called mirror universe is explored again in several episodes of Deep Space Nine. In the first DS9 mirror-themed episode, Kira provides one of the simplest and most elegant explanations of the mirror universe: “the players are the same but everyone seems to be playing different parts.”
Even without the elaborate stage of a mirror universe, enzymes that we think of as “textbook” seem to often play different parts than the ones we take for granted.

Fig. 1. Enzymes: not always playing the role you take for granted (Wikimedia Commons).
Anyone who’s taken a 2nd-year course in biochemistry and physiology- especially those with an appreciation for athletics- are familiar with the enzyme creatine kinase (CK). CK draws upon chemical energy stored in muscle: phosphocreatine, a high-energy phosphate donor. Phosphocreatine is used to phosphorylate ADP and regenerate ATP in instances where oxidative phosphorylation can’t keep up with energy demands, in the following reaction:
Phosphocreatine + ADP  Creatine + ATP
Creatine + ATP
Typically, this occurs within the first few seconds of burst exercise. Basically, CK and phosphocreatine make up the biochemical battery that starts the engine of muscle contraction. Once the engine is running, carbohydrate catabolism and ultimately fat catabolism (that sweet fat burning zone) are the fuels that sustain it (Fig. 2).
Simple, right? Well, if CK can mobilize phosphocreatine reserves to maintain energy levels in burst exercise, why not in other energy-demanding situations?
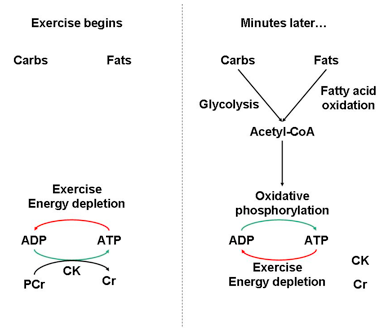
Fig. 2. At the onset of burst exercise, many catabolic processes are inactive and cannot support ATP demand. ATP must therefore be regenerated by phosphocreatine (PCr), a high-energy phosphagen, and the enzyme creatine kinase (CK). CK interconverts PCr, ATP, ADP, and creatine (Cr). Once catabolic processes meet ATP demands (and/or PCr had been depleted), the mode of energy production shifts.
Energy demand… without the exercise
My doctoral research centerpiece was the freeze-tolerant wood frog, Rana sylvatica. Roughly 70% of its extracellular and extra-organ water turns into actual ice. Over the long winter, there’s no heartbeat, no breathing, no brain activity. Once spring rolls around, the frog thaws and resumes its “normal” life. How? One of the main factors is the prevention of intracellular freezing by the use of biological antifreeze. Heavy liver glycogen breakdown is initiated; it’s so powerful that glucose levels in organs and blood can increase to 40-60 times above normal. The thing is, this is a double-edged sword: if glucose needs to be maintained as a pool of cryoprotectant to prevent intracellular freezing, it (for the most part) can’t be used to feed glycolysis and maintain energy levels. Sure, with most metabolic processes shut off as a result of being an ice block ice, the energy demand isn’t terribly high. Still, the levels do need to be kept up to maintain cell viability, as a “life-support” system, as well as for the energetic strain associated with thawing. So where does the needed energy come from?
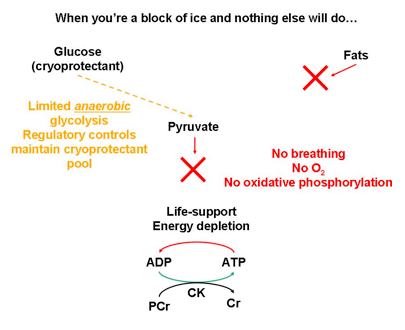
Fig. 3. In freeze-tolerant organisms, lack of respiration and lack of (frozen) blood circulation leads to ischemic hypoxia. Oxygen-dependent fatty acid oxidation and oxidative phosphorylation are not possible, leading to diminished ATP levels. CK can draw upon PCr reserves in certain tissues and organs to maintain energy levels.
In organs and tissues where phosphocreatine reserves are present, such as muscle, frozen frogs can draw upon them (Fig. 3) to maintain energy levels and the all-important life-support. In organs without phosphocreatine, different metabolic strategies come into play.
My focus, when it came to molecular mechanisms of wood frog adaptation, was reversible protein phosphorylation. Surely enough, given that CK is a much-needed enzyme in freeze-tolerance, its kinetic parameters- and enhanced ability to mobilize phosphocreatine energy reserves- are regulated by phosphorylation. As with any other enzyme or pathway regulated by post-translational modification, only certain protein kinases will do the job. I tried phosphorylating CK in vitro by stimulating several major metabolic kinases, but only calcium/calmodulin-dependent protein kinase (Ca2+/CaMK) and adenosine monophosphate-dependent protein kinase (AMPK) had a detectable kinetic effect (Fig. 4).
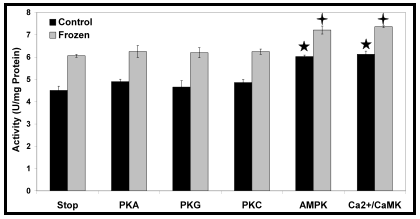
Fig. 4. In Rana sylvatica, several protein kinases were stimulated in vitro in an attempt to phosphorylate CK. However, out of those tested, only two- including the AMP-activated protein kinase (AMPK)- had a detectable effect on CK activity. Replotted from Dieni and Storey, 2009.
Why even bother to mention that AMPK regulates CK activity? I’ve given you enough to chew on, haven’t I? Well, I told you this article was about the surprising roles that an enzyme can sometimes play, and I recently discovered one for CK that surprised even me.
.
.
.
.
Creatine kinase and its role in… insulin secretion and anti-diabetic drugs?
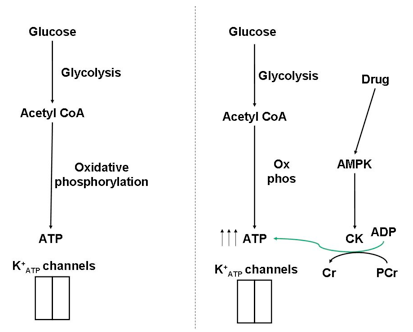
Fig. 5. The import of glucose, and its catabolism in pancreatic β-cells results in an increase in ATP levels, closing K+ATP channels and stimulating insulin secretion. However, this ATP increase may only be transient, resulting in a short insulin release- particularly within diabetics. Anti-diabetic drugs stimulate AMPK, and CK in turn. The localization of CK proximal to K+ATP channels shields the channels against changes in bulk nucleotide concentrations and enhances the insulin release.
First off, as much as I’d like to provide all the necessary background behind the molecular mechanisms of insulin secretion and anti-diabetic drugs (metformin and others), there’s only so much I can put into a BenchFly article. If you’re not very familiar with these mechanisms I’d suggest starting off here for insulin secretion and here for anti-diabetic drugs.
I recently read a paper (Düfer et al., 2010) that describes how anti-diabetic drugs enhance insulin secretion by activating AMPK. But wait… in normal physiology, glucose-stimulated insulin secretion works by catabolising glucose in β-cells, increasing ATP levels. With an increase in ATP, AMP levels would be low and AMPK wouldn’t be active. But by stimulating AMPK via anti-diabetic drugs, AMPK targets can be active in spite of the high glucose levels (and high ATP levels) that accompany insulin secretion. These AMPK targets, of course, include CK. In a more active state, CK can therefore maintain high ATP levels in the microenvironment of K+ATP channels, and enhance insulin secretion beyond the glucose stimulus alone (Fig. 5).
When we think of enzymes, we often think of them in a very narrow role that may only represent a small fraction of their physiological potential. What moments ago may have seemed to you like an enzyme that works only within the first few seconds of jogging or bench-pressing, actually keeps an organism alive during the months it spends frozen, and works with anti-diabetic drugs to enhance insulin secretion. If you poke your head around metabolism, you’ll see that creatine kinase is hardly unique in this respect.
What enzymes have you come across that have roles outside of the textbook norm?
.
.
References
- Dieni, C.A. and Storey, K.B. (2009) Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B 152: 405-412.
- Düfer, M., Noack, K., Krippeit-Drews, P. et al. (2010) Activation of the AMP-activated protein kinase enhances glucose-stimulated insulin secretion in mouse β-cells. Islets 2: 156-163.
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
.
.
]]> Not long ago, I was tasked with assaying for levels of glycogen in animal tissue. In most labs nowadays, glycogen is assayed by enzymatically hydrolyzing glycogen down to its component glucose monomers, and then enzymatically oxidizing that glucose in turn, producing hydrogen peroxide which can then react with a number of chromogens to produce a colored product.
Not long ago, I was tasked with assaying for levels of glycogen in animal tissue. In most labs nowadays, glycogen is assayed by enzymatically hydrolyzing glycogen down to its component glucose monomers, and then enzymatically oxidizing that glucose in turn, producing hydrogen peroxide which can then react with a number of chromogens to produce a colored product.
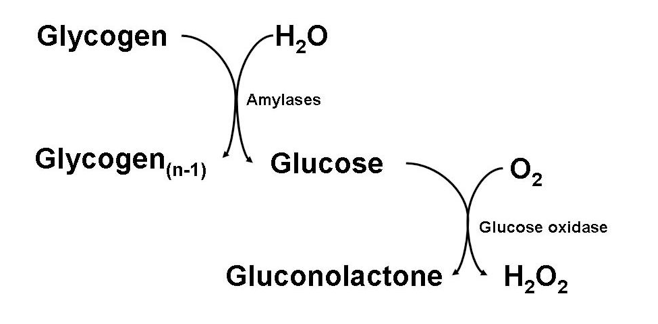
The problem with assaying glycogen in this manner, however, is that any baseline glucose present in your tissue extract will also be oxidized, produce hydrogen peroxide, and react with your chromogen. This can cause quite a high and significant blank, especially when your tissue handling and storage may not be as quick as it should, and some glycogen hydrolysis occurs in tissues post-collection.
A very good practice of avoiding this glucose blank, even within tissues that have been expertly handled, is to homogenize and process tissue samples in such a way that polymerized glycogen is precipitated, whereas monomeric glucose remains in solution. After the appropriated centrifugations and washes, glycogen is re-dissolved, now free of any interfering glucose. This method is to digest tissue samples in a strongly alkaline solution at high temperature: 30% potassium hydroxide (KOH), 95°C, 30 minutes (or more!) seems to be the norm. Needless to say, boiling your tissue samples in caustic solution is not a very common thing to do. It therefore was somewhat expected when I was asked an important question: under these harsh conditions, what’s to stop glycogen itself from being hydrolyzed along with a plethora of other biomolecules present in animal tissue?
I knew as soon as the question was asked that glycogen couldn’t possibly be hydrolyzed- at least not to a significant extent. Why? Well, if it were, then this sort of thing wouldn’t be published in the literature countless times, over many years. Okay, but other than standing on the shoulders of these published giants- seriously, chemically speaking- why isn’t glycogen hydrolyzed under the very same conditions that are used to dispose of entire human bodies in oh so many murder mysteries? Got lye?
First of all, how is glycogen normally broken down? And by “normally” I mean its metabolism within living organisms?
Intracellular glycogen metabolism
The breakdown of glycogen within cells has always fascinated me. Normally, when I think of the product of many glucose monomers linked together, I expect glucose itself, as shown in the assay scheme above. Instead, the intracellular “hydrolysis” product of glycogen is glucose-1-phosphate. Where did this phosphate come from?
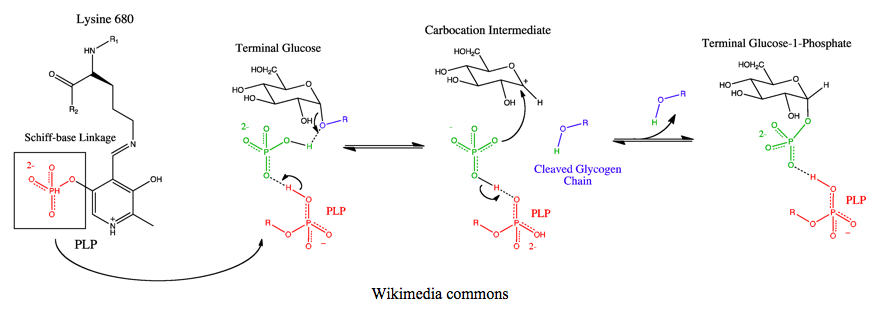
That’s because glycogen hydrolysis within cells isn’t a true hydrolysis. It’s a phosphorolysis. With the help of the pyridoxal phosphate (PLP) cofactor in its catalytic core, the glycosidic bond between two glucoses seemingly effortlessly collapses and scoops up a proton from inorganic phosphate, leaving behind glycogen reduced by one glucose monomer (glycogen(n-1)) and an oxonium glucose ion; a positively charged glucose carbocation stabilized by an electron lone pair from its neighboring oxygen. The remaining phosphate then extends a lone pair from any of its three deprotonated oxygens, bonding with the carbocation, creating the glucose-1-phosphate product. Glucose-1-phosphate is then converted to glucose-6-phosphate by phosphoglucomutase, allowing it to be shuttled into any number of the fates of glucose; dephosphorylation and export as free glucose, glycolysis, or oxidation in the pentose phosphate pathway.
Extracellular glycogen metabolism (digestion)
Outside of cells (such as within the digestive tract), glycogen is metabolized by a family of enzymes called the glycoside hydrolases, which include the amylase isozymes, as well as lyzosyme. This is actually quite a separate family of enzymes from glycogen phosphorylase; glycogen phosphorylase falls into the glycosyltransferases (EC 2.4) whereas glycoside hydrolases are within the sugar hydrolases (EC 3.2).
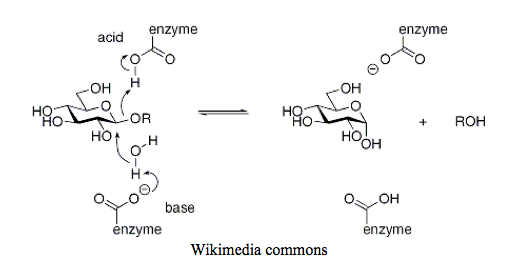
The mechanism by which glycogen hydrolysis occurs here has some similarities, and other differences. A cofactor like PLP is not involved, but rather the direct participation of amino acid side chains that are protonated and deprotonated. A deprotonated side chain, like aspartate or glutamate, abstracts a proton from water to generate a hydroxide nucleophile. This nucleophile then attacks the 1-carbon of the glycosidic bond, ousting the remaining glycogen(n-1), taking a proton from another amino acid side chain with it. All in one fluid step- at least, that’s how this figure shows it.
My favourite organic chemistry textbook (author’s note: it’s Organic Chemistry by Paula Yurkanis Bruice) depicts a slightly different catalytic mechanism for lysozyme, clearly showing two distinct steps with greater similarity to glycogen phosphorylase. The first is the collapse of the glycosidic bond and protonation of the remaining glycogen(n-1) polymer, as well as the formation of the oxonium ion on the glucose monomer. The second is the generation of hydroxide from water, and transfer of this hydroxide to the carbocation.
Chemical considerations
How does that scenic enzymatic route help us? We already know that enzymes do pretty extraordinary things at near-neutral pH that would otherwise require extreme conditions to achieve outside of a protein core. That tells us nothing new. Or does it?
The mechanism of glycogen phosphorylase, above, required the protonation of the electrons forming the glycosidic bond in order for glycogen to break away from a glucose monomer. The mechanism of the glycoside hydrolases- depending on which figure you look at and whether you believe it’s a 1-step or 2-step mechanism, either simultaneously or pre-emptively also requires protonation of the glycosidic bond.
Of course it does (I said after beating my head against a wall for several days)! In organic chemistry terms, glucose is an aldehyde in its linear form. Its cyclization is really the formation of a hemiacetal. Its linkage to another sugar to form a disaccharide (or longer) is the formation of an acetal. While the interconversion of the cyclic hexose hemiacetal is spontaneous, the full acetal needs something a bit more- something that we would expect from acetal equilibrium with any given organic molecule and not just glucose-glycoside acetals: acidic conditions.
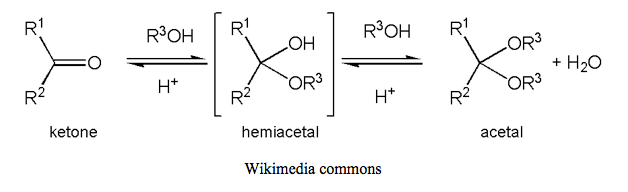
.
What biochemical polymers can be digested by alkaline hydrolysis?
- Triglycerides: the ester linkages between the central glycerol and each of the three bound fatty acids in a triglyceride molecule are hydrolyzed by hydroxide nucleophiles.
- Proteins: the amide bonds between amino acids are hydrolyzed. Several amino acids themselves are also destroyed under these conditions.
- Nucleic acids: RNA but not DNA is hydrolyzed in alkaline conditions. Why RNA and not DNA? The actual nucleophile that does the hydrolyzing is the 2’OH, which is generated by deprotonation from the alkaline conditions. This then results in an intramolecular hydrolysis of the phosphodiester linkages. No 2’OH, no hydrolysis.
.
Yet, glycogen remains intact under harsh alkaline conditions. When initially asked why that is, I was uncertain. It took looking through the catalytic mechanisms of two enzyme families to gain some insight as to why…
Ultimately, you know what was the biggest clue? 2nd year undergrad organic chemistry. Let it never be said that “we’ll never need this stuff again.”
.
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
.
.
]]> If I were to ask you- yes, all of you out there- what you thought was the cornerstone method for assessing changes in metabolism, and metabolic regulation as a whole, what would your answer be?
If I were to ask you- yes, all of you out there- what you thought was the cornerstone method for assessing changes in metabolism, and metabolic regulation as a whole, what would your answer be?
.
Your choices
All those who were thinking of using RT-PCR to measure changes in gene transcription, raise your hand. Hey, you too, hiding back there- I know you’re a huge PCR nerd!
Now, those of you who were planning on using western blots to see changes occurring at the translational level, raise your hand. But wait; keep them up for a second. Which antibodies were you planning on using? Was it just a pan-specific antibody that would detect your protein no matter what state it were in? If so, you can go ahead and put your hand down. But, those of you who were planning on using an antibody specific to a post-translational modification- something like phosphorylation, acetylation, nitrosylation, glycosylation, or any of the others- you’ve got my attention.
One last one: were any of you planning on assaying any enzyme activity? Maybe using a colourimetric or spectrophotometric method? Great!
That lazy friend of yours…
Remember on Seinfeld when Kramer formulates a plan to completely reconfigure his apartment? He intends to get rid of all the furniture and just build a bunch of levels… with carpeting, and a lot of pillows, like ancient Egypt. Jerry figures that it won’t get done within the month that Kramer allots himself, and- surely enough- Kramer doesn’t even attempt the levels.

Cosmo Kramer. If this guy designed our cells, we'd all be in big, big trouble.
We all have them: the friends who make up big plans that never quite seem to materialize. What’s unfortunate, too, is that it’s not really even an all-or-nothing concept. There are various degrees to which Kramer’s plans might not have panned out. What if he hadn’t just dropped the idea entirely, but instead would’ve actually taken a few steps towards realizing his dream of levels? Let’s say he had bought all the lumber- would he have properly measured twice, cut once? If he had hammered together all the pieces and made the entirety of these wooden levels- would he have properly carpeted all of them? And if he had laid down the finest carpets and rugs- would he have made the effort to find the appropriate pillows to go with them? There were not just one, but many steps that Kramer would’ve needed to get through before his levels were truly complete.
Kramer told Jerry that he didn’t draw up any plans, that the blueprint was entirely in his head. Similarly, the blueprint for any would-be metabolic “levels” are stored right there in DNA. DNA stores the code for any molecular mechanism of metabolic regulation that could ever be needed. Just like building those levels, though, there are many steps to get through before any of those blueprints become functional. DNA could just sit there, tightly coiled around its histones, not doing terribly much- like that blueprint locked away in Kramer’s brain. Histones may be acetylated, unlocking that DNA- but would it be unwound by helicase? DNA may be transcribed into RNA- but will that RNA be processed before it is degraded? Capped, polyadenylated mRNA may exit into the cytoplasm- but would it be translated by ribosomes? And full-length, folded protein may be synthesized- but would it be active? After all, proinsulin isn’t that useful (or friendly, from an immunogenic point of view), and the unphosphorylated b form of glycogen phosphorylase isn’t especially active when there’s no AMP to be seen.
Where the (bio)chemistry happens
As an enzymologist, of course I’m biased. But as anyone in the biological sciences will easily admit, active enzymes are where the chemistry of life takes place. Without those glorious biological catalysts pushing those reactions along, life as we know it simply wouldn’t exist. Sure, many would argue that ribozymes have catalytic powers of their own- but they can’t compare to the complexity and vastness of their protein cousins.
There is, of course, absolutely nothing wrong with the steps that come before functional enzymes! DNA, transcription, mRNA, translation, degradation- all of these are golden within the Central Dogma. These are perfectly viable metabolic control sites, studied and focused upon by countless scientists around the world. The take home message that I’ve been thrusting upon you, overall, is that while steps before active enzymes have so much potential, it is those active enzymes themselves that have the function.
After all- earlier, I mentioned a few epigenetic control mechanisms. Acetylases to acetylate histones and unlock DNA? Helicase to unwind the double helix, and allow RNA polymerase access? RNA polymerase itself? All of these are enzymes… enzymes that make the biochemistry happen.
Did I mention that 2011 is the International Year of Chemistry?
What do you say? What is your bread and butter of metabolic control? Those of you who aren’t enzyme-centric, feel free to knock me on my posterior and put me in my place!
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
.
.
]]> A few weeks back, I was sending tweets back and forth with Alan. It started when Alan had asked, in reference to a previous BenchFly blog post, whether it was better to focus on a specific research field, or to be a jack of all trades. I joked that I preferred to focus on what I liked to think of as “interdisciplinary research,” a professional (and slightly nerdy) way of portraying one’s self as a jack of all trades. This prompted Alan to say that as a Chemical Biologist, one could do just that.
A few weeks back, I was sending tweets back and forth with Alan. It started when Alan had asked, in reference to a previous BenchFly blog post, whether it was better to focus on a specific research field, or to be a jack of all trades. I joked that I preferred to focus on what I liked to think of as “interdisciplinary research,” a professional (and slightly nerdy) way of portraying one’s self as a jack of all trades. This prompted Alan to say that as a Chemical Biologist, one could do just that.
I liked Alan’s answer. I’ve always been interested in the field of Chemical Biology. One of the main reasons it has captivated my interest is because, really, I have no idea what Chemical Biology is; but it seems to bundle together quite a bit of methodology and concepts! Certainly, I can go onto a generic site like Wikipedia and read their article on Chemical Biology. It appears to be a rich, fascinating field full of enzymology, proteomics, and protein phosphorylation.
Hang on a second though…
I’ve spent the past 7 years working on enzymology, proteomics, and protein phosphorylation. However, I’m not a Chemical Biologist. Rather, I’m a Biochemist. My undergraduate diploma even says so (that said, my graduate diploma simply says Chemistry).
Something else I’ve always found amusing about Chemical Biology is its very name; the way it is essentially an inversion of Biological Chemistry, the long-form version of Biochemistry.
With that, getting back to our Twitter-conversation, I asked Alan if he thought that there was a difference between Chemical Biology and Biological Chemistry. He said that he didn’t feel there was- it was more splitting hairs than anything else. He did add, however, that there is a difference between Chemical Biology and Biochemistry.
Whoa! Back up two paragraphs there…
No difference between Chemical Biology and Biological Chemistry, but yes, a difference indeed between Chemical Biology and Biochemistry? Aren’t Biological Chemistry and Biochemistry one and the same? Is Biochemistry not simply a portmanteau of Biology and Chemistry? Biology & Chemistry… Biological Chemistry… Bio-Chemistry… BioChemistry… Biochemistry… are there any differences here?
If nothing else, it is a fun debate. Whether it is the proverbial splitting of hairs, or whether these are concrete, diametrically-opposed fields, weeks later I am still left to wonder whether there is a distinct difference between Biochemistry, Biological Chemistry, and Chemical Biology. Has science in the 21st century generated a complex Venn diagram of sub-fields? Ah, the good old days when the only three sciences I had to be concerned with were Biology, Chemistry and Physics.
.
.
Note: There is a poll embedded within this post, please visit the site to participate in this post's poll..
.
Chris is originally from Montreal, and defines himself (perhaps ominously) as a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
What are your thoughts- can you bring any clarity to the debate?
.
.
]]>