Make Up Your Mind! When Phosphorylation Turns Enzymes “ON” or “OFF”
by
 Previously, I told you about how phosphorylation can turn some enzymes on, and other enzymes off. In my new Enzyme Corner article here on BenchFly, as I’m quite fond of doing I throw yet another monkey wrench into the machine. Today, we discuss how some enzymes can be both activated and inactivated by phosphorylation.
Previously, I told you about how phosphorylation can turn some enzymes on, and other enzymes off. In my new Enzyme Corner article here on BenchFly, as I’m quite fond of doing I throw yet another monkey wrench into the machine. Today, we discuss how some enzymes can be both activated and inactivated by phosphorylation.
Textbook Protein Phosphorylation
When I was an undergraduate in 2nd year biochemistry, one of the first enzymes controlled by protein phosphorylation that I learned about, was the liver isozyme of pyruvate kinase. As the last enzyme of glycolysis, and a step that irreversibly converts phosphoenolpyruvate into pyruvate, it’s a step that needs to be tightly controlled in physiological situations where glucose may be limiting and should preferentially be diverted to other tissues like muscle and brain. When blood glucose is high, the insulin signal is active, which activates/targets protein phosphatase 1 (PP1) to its substrates. PP1 dephosphorylates pyruvate kinase, turning it on. After a time, blood glucose drops. The glucagon or epinephrine signal becomes dominant, which leads to the activation of protein kinase A (PKA). PKA phosphorylates and inactivates pyruvate kinase. Take a look at Fig. 1 for a schematic representation.
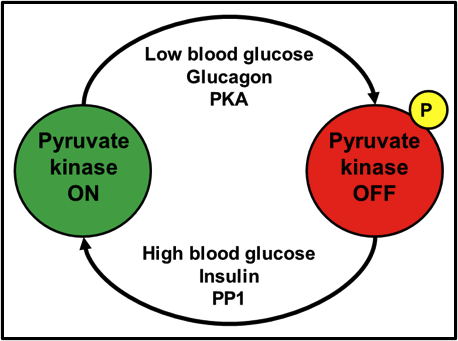
Fig. 1. Activation of pyruvate kinase by dephosphorylation; inactivation by phosphorylation.
This textbook explanation of the phosphorylation-mediated activation/inactivation of an enzyme, albeit accurate, is quite simplistic. For a 2nd year biochemistry student, it really is all they need to know at the time; for those of us who move beyond the 2nd year of our undergraduate studies, however, this type of textbook explanation (and many others across many niches in the biological sciences) engrains within us the perception that an enzyme has one and only one phosphorylation site, and that phosphorylation will either turn an enzyme on or off.
GSK-3: A Servant with Many Masters
Previously, I told you about glycogen synthase kinase-3 (GSK-3), a protein kinase with many substrates- most notably glycogen synthase. However, as is the case with many protein kinases, they have protein kinase kinases of their own that regulate them- such is the way that the hierarchy of signal transduction has evolved.
GSK-3 has two well-known isozymes: GSK-3α and GSK-3β. There is a homologous serine on both these isozymes; Ser9 on GSK-3α and Ser-21 on GSK-3β. Phosphorylation of this serine by an upstream kinase, such as Akt, inactivates GSK-3 and prevents it in turn from phosphorylating its own downstream targets. Interestingly enough, though, there is another site- a tyrosine residue of note. Whereas Akt phosphorylation of Ser9/21 shuts GSK-3 down, phosphorylation of that tyrosine, by protein kinases other than Akt, activates GSK-3 even more.
Ser9/21: Catalytic Site Inhibition
Part of understanding why phosphorylation of one site turns GSK-3 off and phosphorylation another site turns it on (even more), is understanding what each phosphorylation does to the structural-functional relationship of GSK-3.

Fig. 2. A plug chained to a bathtub faucet fits into the bathtub drain. All are connected; essentially, one part of the structure is used to block a hole in another part of the very same structure – Designer Plumbing.
Imagine you’ve had a long day at work and you’re a bath type of person. You turn on the water faucet in your bathtub. If you leave things the way they are, that water is simply going to flow into the drain and downward- you’re going to be waiting a heck of a long time for your tub to fill up, won’t you? So, what do you do? For the sake of proper imagery in this analogy, imagine you have an old-school bathtub with a long chain that connects your faucet to a plug. You put the plug into your drain. Now that the plug is exactly where the water needs to go, it can’t get in! Effectively, by using a plug that was chain-linked to the faucet, you used a piece of bathtub’s own structure to plug a cavity into which something (the water) would naturally fit (Fig. 2).
You can likely see where this is going: Ser9/21 is part of the GSK-3 structure that has the flexibility and potential to curve back inward, all the way to the catalytic core. Since the catalytic core uses ATP to place a phosphate onto a serine or a threonine, a phosphoserine precisely blocks any potential substrate to be phosphorylated from entering the catalytic core, shutting GSK-3 down. Take a look at Fig. 3.
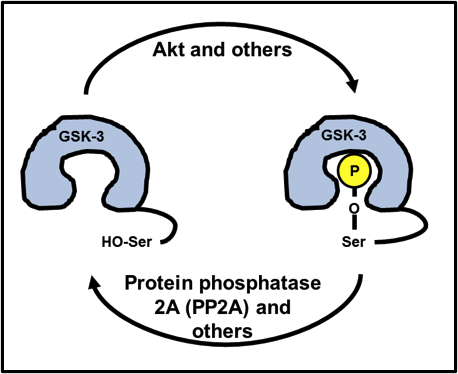
Fig. 3. Inhibition of GSK-3 by Ser9/21 phosphorylation. A part of GSK-3’s own structure folds back into its catalytic core, blocking substrate phosphorylation.
Open Arms (no, not the Journey song): Tyr279/216
Where Ser9/21 phosphorylation inactivates GSK-3, phosphorylation of Tyr279 in GSK-3α or Tyr216 in GSK-3β, activates it. Admittedly, after a thorough literature search, the Tyr-mediated activation of GSK-3 is seemingly less studied and less clear than the Ser-mediated inactivation. I liken Tyr279/216 to a relationship with a loved one. When you need a hug from them and reach in, chances are that a loved one will never push you away (if they do, that’s another issue entirely!), and they’ll hug you back. What makes that hug so much sweeter, though, is when their arms are open and they’re inviting you in for a hug, even before you reach out to them. Tyr279/216 phosphorylation rotates said tyrosine outward, away from the substrate-binding region of the catalytic core, opening up the substrate-binding region even more. Essentially, a phospho-Tyr279/216 GSK-3 is inviting its substrates in for a great big bear hug (Fig. 4)!
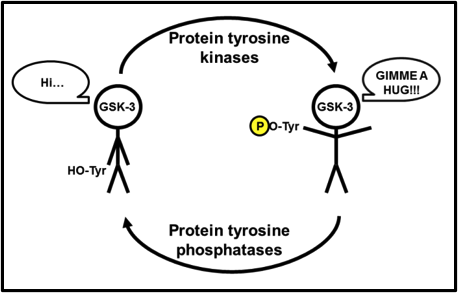
Fig. 4. Further activation of GSK-3. GSK-3 is active even without Tyr279/216 phosphorylation, but tyrosine phosphorylation opens up the substrate binding region of the catalytic core, allowing for greater substrate access.
But… who wins?
What if both Ser9/21 and Tyr279/216 are phosphorylated at the same time? Who wins out: the bathtub plug or the bear hug? Sadly, no matter how much a GSK-3 invites a substrate in for a hug, if the serine or threonine of said substrate can’t reach the catalytic core to be phosphorylated, because phospho-Ser9/21 is blocking the way, the biochemistry of protein phosphorylation simply can’t proceed. Therefore, Ser9/21 inactivation wins out over Tyr279/216 activation. Fig. 5 illustrates the relationships.
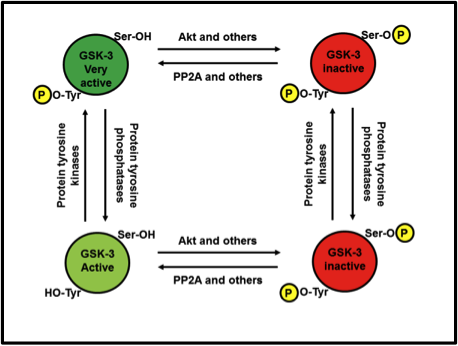
Fig. 5. Relationship between Ser9/21 phosphorylation and Tyr279/216 activation. Tyrosine phosphorylation can turn an active GSK-3 into a very active GSK-3. However, regardless of whether tyrosine is or isn’t phosphorylated, as soon as serine is phosphorylated, it’s game over.
Further reading
While admittedly a dozen years out of date, very few present-day reviews can hold a candle to this review article: Cohen, P. (2000) The regulation of protein function by multisite phosphorylation – a 25 year update. Trends Biochemical Sci. 25: 596-601. Written by the venerable guru of protein phosphorylation, Sir Philip Cohen at the University of Dundee, this piece deals specifically with proteins that are regulated by multiple phosphorylation sites. Highly recommended for more advanced reading!
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
Have any kinase-related questions for Chris?
.


Brian
wrote on September 24, 2012 at 3:58 am
In Figure 5, isn’t the lower right GSK-3 supposed to be dephosphorylated at the tyrosine site?
Christopher Dieni
wrote on December 15, 2013 at 1:33 pm
You're certainly right, it is. Sorry about that.
Soe
wrote on December 12, 2013 at 5:06 pm
Can you give a reference/citation for figure 5?
Christopher Dieni
wrote on December 15, 2013 at 1:22 pm
Hi Soe. It's not really something that can be found in a single specific reference- mostly it's something I aggregated from various literature sources.
Before going any further though I will point out, as indicated in a comment above, that there is an error in the figure; the GSK-3 at the bottom-right should only be phosphorylated at the serine, not the tyrosine.
Here's a few references you might want to check out though: http://www.pnas.org/content/97/22/11960.full http://www.ncbi.nlm.nih.gov/pubmed/20652371 http://www.ncbi.nlm.nih.gov/pubmed/14570592 http://www.hindawi.com/journals/ijad/2011/479249/
Christopher Dieni
wrote on December 15, 2013 at 1:22 pm
Hi Soe. It's not really something that can be found in a single specific reference- mostly it's something I aggregated from various literature sources.
Before going any further though I will point out, as indicated in a comment above, that there is an error in the figure; the GSK-3 at the bottom-right should only be phosphorylated at the serine, not the tyrosine.
Here's a few references you might want to check out though: http://www.pnas.org/content/97/22/11960.full http://www.ncbi.nlm.nih.gov/pubmed/20652371 http://www.ncbi.nlm.nih.gov/pubmed/14570592 http://www.hindawi.com/journals/ijad/2011/479249/
Christopher Dieni
wrote on December 15, 2013 at 1:22 pm
Hi Soe. It's not really something that can be found in a single specific reference- mostly it's something I aggregated from various literature sources.
Before going any further though I will point out, as indicated in a comment above, that there is an error in the figure; the GSK-3 at the bottom-right should only be phosphorylated at the serine, not the tyrosine.
Here's a few references you might want to check out though: http://www.pnas.org/content/97/22/11960.full http://www.ncbi.nlm.nih.gov/pubmed/20652371 http://www.ncbi.nlm.nih.gov/pubmed/14570592 http://www.hindawi.com/journals/ijad/2011/479249/
Christopher Dieni
wrote on December 15, 2013 at 1:22 pm
Hi Soe. It's not really something that can be found in a single specific reference- mostly it's something I aggregated from various literature sources.
Before going any further though I will point out, as indicated in a comment above, that there is an error in the figure; the GSK-3 at the bottom-right should only be phosphorylated at the serine, not the tyrosine.
Here's a few references you might want to check out though: http://www.pnas.org/content/97/22/11960.full http://www.ncbi.nlm.nih.gov/pubmed/20652371 http://www.ncbi.nlm.nih.gov/pubmed/14570592 http://www.hindawi.com/journals/ijad/2011/479249/
Soe
wrote on December 12, 2013 at 5:06 pm
Can you give a reference/citation for figure 5?