Nanoparticles and Proteins, Part I
by
I’ve been absent for far, far too long, and before anything else, I’ll need to apologize for that. This new Enzyme Corner article will thus serve a dual-function: 1) telling a new enzyme/protein story, which is far overdue, and 2) bringing you up to speed on what I’ve been up to lately.
.
For those who came in late…
Because it’s been ages since I last wrote for BenchFly, before I tell you what I’m up to now, let me give you a bit of a 5-year summary of where I’ve been and where I am. Five years ago, in June 2008, I was wrapping up my PhD thesis in the Storey Lab at Carleton University in Ottawa, where I studied, in the grand scheme, the molecular mechanisms of cryobiosis; I looked at important metabolic and signaling enzymes in the freeze-tolerant frog, Rana sylvatica, the hibernating Richardson’s ground squirrel, Spermophilus richardsonii, and sometimes poked around with other animals too. At the end of August 2008, I then moved to the Benkovic Lab at Penn State, where I researched for the following 2 years the regulatory controls involved in the reversible assembly of the purine biosynthetic enzyme complex in HeLa cells. Following this, I returned to Montreal, where in August 2010 I began a fellowship known as the Natural Sciences and Engineering Research Council-Industrial Research and Development Fellowship (NSERC-IRDF) at a startup biotech company called Micropharma Ltd. Here, I worked on various aspects of disease-targeted probiotics that were rich in the activity of key metabolic enzymes. I also contributed to the development of an enzyme-driven, nitric oxide-generating medical dressing with antimicrobial and anti-biofilm activity.
Now
Since July 2012, I’ve been at Mount Allison University, the Maclean’s magazine-ranked top primarily-undergraduate university in Canada for 16 out of the past 22 years. I hold the position of the Margaret and Wallace McCain Postdoctoral Fellow, which is actually a bit closer to faculty than the word “postdoctoral” would have you believe. I teach a half-course load, having taught a 4th-year biochemistry course called Immunochemistry last Fall semester, and another 4th-year biochemistry course called Signal Transduction over the Winter semester. For this coming Fall semester, I’m developing a brand-new, never-before-taught 3rd-year biochemistry course called Toxicology.
I also get to participate in research activities, and though the limited-term nature of the 2-year position doesn’t allow me a research infrastructure of my own, I do get to collaborate with faculty who share interests similar to my own. Best of all, as of May, I am now a full-fledged supervisor of my first two very own undergraduate honors thesis students.
As far as research goes, I’m once again back in the domain of comparative biochemistry and physiology, relying on animal models and focusing on metabolic regulation of stress physiology, with a bit of twist: this time around, the stressor is not cold or other naturally-occurring environmental phenomenon, but rather, a man-made toxicant. That toxicant is the spherical nanoparticle.
Nanoparticles
Finally, on to the science! There’s a lot I could say about nanoparticles, but as a biochemist/physiologist, even I’m forced to admit that much of it could be better-said by a materials scientist. For the interests of this article, in a nutshell a nanoparticle can be composed of a variety of elements, both metallic and/or nonmetallic. They are colloidal assemblies of that/those element(s) at the nanoscale- smaller than micrometers but larger than picometers. Because of the way these nanomaterials are assembled, their surface area to volume ratio, and their varieties of surface coatings or functionalizations, they have unique properties; the properties of the colloids are different from the ionic or molecular versions of their constituents (also known as the “bulk” constituents), and also different from the macroscale, solid forms of these materials as well.
Overall, because nanoparticles are roughly on the same scale of a protein’s size, they tend to interact with proteins… and not in a good way. In order to keep nanoparticles in suspension and keep them from agglomerating (or “clumping”) their surface coatings are often charged, allowing them to accumulate bilayers of ions from whatever fluid (biological or otherwise) in which they happen to be suspended (see Figure 1). When they are, indeed, suspended in biological media, the ions they accumulate are not only simple ionized atoms (Na+, K+, Cl–, etc.), but also proteins replete with their own electrostatic surfaces (aspartate, glutamate, lysine, arginine, histidine). A nanoparticle will eventually gather a collection of proteins interacting with its surface, which is becoming more prevalently known in the field of nanotoxicology as a “protein corona.” Proteins can interact reversibly with the nanoparticle surface, associating and dissociating if the interaction is weak enough. Given enough time and the right proteins, however, some proteins may adsorb more strongly to the nanoparticle surface than others, forming a tighter and overall a more irreversible association.
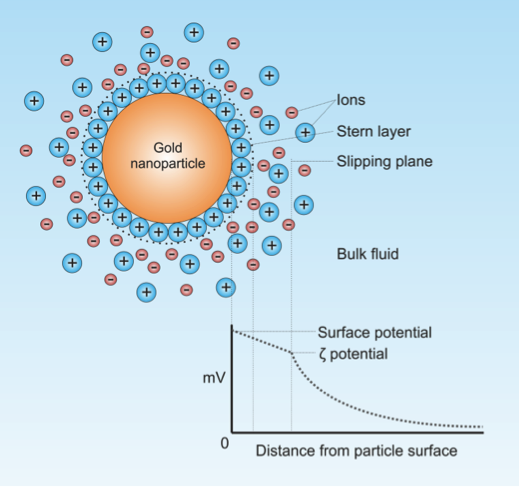
Fig 1. Depiction of a gold nanoparticle and the layers of ions formed around it that allows it to remain in suspension. (Source: Wikipedia, Creative Commons)
The protein
Many scientists in the field of nanoscience study the effects of these protein corona from the perspective of the nanoparticle. How does this change the surface physical and chemical properties of the nanoparticle? How does this affect the disposition (distribution, biotransformation, and excretion) within a living organism? And so on and so forth. However, these questions essentially ignore the co-star of the nanoparticle-protein interaction. In other words, what about the protein?
Well… think about a protein in its native conformation, in its native (and unperturbed) environment. Now… think about that same protein “stuck” (in some way, shape, or form) to a nanoparticle. Through electrostatic interactions, through Van der Waals forces, perhaps to other proteins that are also associated with the nanoparticle like links on a chain, this association is clearly not a natural one, as ionic or other side-chains on the protein are stretched or compacted in their complex interaction with a nanoparticle.
Needless to say, while a protein may not be outright denatured by its interactions with a nanoparticle, it is most definitely not in what we consider to be a “normal,” native conformation.
Structural-__________ relationships
With most types of molecules or biomolecules that we study in the biological sciences, structure has a direct impact on function. And while the primary amino acid sequence doesn’t change, for all intents and purposes conformational changes are structural changes. If a protein is incorporated into a nanoparticle’s protein corona and its conformation is perturbed, you can make a fairly safe bet that its function is similarly perturbed (see Figure 2).
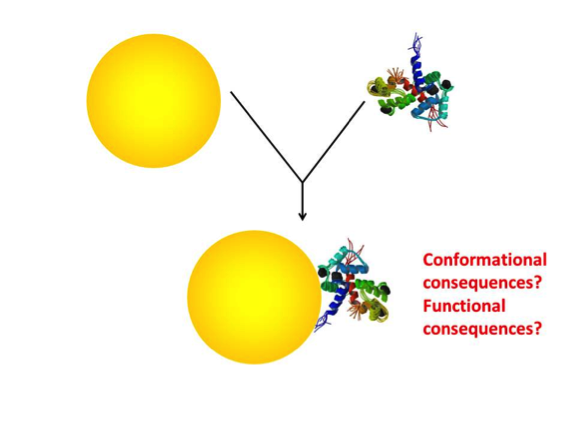
Fig 2. Depiction of a gold nanoparticle and a protein forming an association (the gathering of a “protein corona”) and the pondering of the associated conformational and functional consequences to the protein.
What function, however, are we talking about?
Well, that depends on the protein(s) that is/are interacting with the nanoparticle. Virtually any protein can interact with a nanoparticle, to a weaker or stronger degree, based on the localization of that nanoparticle within an organism. Thus opens the wide field of molecular mechanisms of nanotoxicology.
For instance, are we talking about:
- “Carrier” proteins of otherwise-insoluble ions biomolecules in the plasma?
- Proteins of the innate and adaptive immune systems in the plasma?
- Structural proteins in tissue and cell membranes?
- Catalytic proteins (i.e. enzymes) in mucus, bile, interstitial fluid, plasma or just about anywhere else?
- And then… if a nanoparticle happens to cross the plasma membrane and make its way into the intracellular environment… what happens to membrane, cytosolic, organellar, and nuclear components, protein or otherwise?
Pandora’s box
All the above focused on, for lack of a better term, the physical aspects of the interactions between nanoparticles and proteins. We’ve said nothing so far about the chemical properties of nanoparticles, of which there are many, particularly their generation of reactive oxygen species.
Next time, we’ll look at some more specific mechanistic examples.
 Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa, State College (Penn State), and finally back to Montreal’s biotech industry. Presently, he is the Margaret and Wallace McCain Postdoctoral Fellow at Mount Allison University in Sackville, New Brunswick, Canada. He is researching the role of protein phosphorylation in physiological stress, and teaching 4th-year undergraduate courses in Biochemistry.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa, State College (Penn State), and finally back to Montreal’s biotech industry. Presently, he is the Margaret and Wallace McCain Postdoctoral Fellow at Mount Allison University in Sackville, New Brunswick, Canada. He is researching the role of protein phosphorylation in physiological stress, and teaching 4th-year undergraduate courses in Biochemistry.
What, you missed one of Chris’ previous articles?! Don’t worry, you’re just a click away.
- Make Up Your Mind! When Phosphorylation Turns Enzymes “ON” or “OFF”
- Phosphoproteins: Where’s the “ON” Switch?
- Is There Really Science in the Twitterverse?
- Know Your Role: Enzymes and Their Unexpected Physiological Functions
- Tearing It Up: Glycogen and Its Chemical and Biochemical Breakdown
- Enzymes and the Problem with Cosmo Kramer’s Levels
- What’s in a Name?
.


