Know Your Role: Enzymes and Their Unexpected Physiological Functions
by
 Did you ever see the episode “Mirror, Mirror” in the original Star Trek series? In a nutshell: Kirk, McCoy, Scotty and Uhura get sent- via one of many transporter accidents- to a parallel universe where people and places are the same, but their history and behaviour are strikingly different. Instead of the enlightened Federation, there’s the barbaric Terran Empire its subjugated alien species. Rather than earning promotions, crew members of the ISS Enterprise advance by assassinating their superiors. And in the boldest fashion statement of all, Spock is rocking a beard. The so-called mirror universe is explored again in several episodes of Deep Space Nine. In the first DS9 mirror-themed episode, Kira provides one of the simplest and most elegant explanations of the mirror universe: “the players are the same but everyone seems to be playing different parts.”
Did you ever see the episode “Mirror, Mirror” in the original Star Trek series? In a nutshell: Kirk, McCoy, Scotty and Uhura get sent- via one of many transporter accidents- to a parallel universe where people and places are the same, but their history and behaviour are strikingly different. Instead of the enlightened Federation, there’s the barbaric Terran Empire its subjugated alien species. Rather than earning promotions, crew members of the ISS Enterprise advance by assassinating their superiors. And in the boldest fashion statement of all, Spock is rocking a beard. The so-called mirror universe is explored again in several episodes of Deep Space Nine. In the first DS9 mirror-themed episode, Kira provides one of the simplest and most elegant explanations of the mirror universe: “the players are the same but everyone seems to be playing different parts.”
Even without the elaborate stage of a mirror universe, enzymes that we think of as “textbook” seem to often play different parts than the ones we take for granted.

Fig. 1. Enzymes: not always playing the role you take for granted (Wikimedia Commons).
Anyone who’s taken a 2nd-year course in biochemistry and physiology- especially those with an appreciation for athletics- are familiar with the enzyme creatine kinase (CK). CK draws upon chemical energy stored in muscle: phosphocreatine, a high-energy phosphate donor. Phosphocreatine is used to phosphorylate ADP and regenerate ATP in instances where oxidative phosphorylation can’t keep up with energy demands, in the following reaction:
Phosphocreatine + ADP ↔ Creatine + ATP
Typically, this occurs within the first few seconds of burst exercise. Basically, CK and phosphocreatine make up the biochemical battery that starts the engine of muscle contraction. Once the engine is running, carbohydrate catabolism and ultimately fat catabolism (that sweet fat burning zone) are the fuels that sustain it (Fig. 2).
Simple, right? Well, if CK can mobilize phosphocreatine reserves to maintain energy levels in burst exercise, why not in other energy-demanding situations?
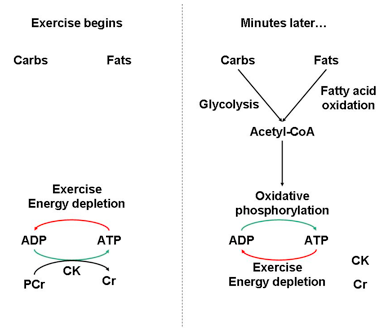
Fig. 2. At the onset of burst exercise, many catabolic processes are inactive and cannot support ATP demand. ATP must therefore be regenerated by phosphocreatine (PCr), a high-energy phosphagen, and the enzyme creatine kinase (CK). CK interconverts PCr, ATP, ADP, and creatine (Cr). Once catabolic processes meet ATP demands (and/or PCr had been depleted), the mode of energy production shifts.
Energy demand… without the exercise
My doctoral research centerpiece was the freeze-tolerant wood frog, Rana sylvatica. Roughly 70% of its extracellular and extra-organ water turns into actual ice. Over the long winter, there’s no heartbeat, no breathing, no brain activity. Once spring rolls around, the frog thaws and resumes its “normal” life. How? One of the main factors is the prevention of intracellular freezing by the use of biological antifreeze. Heavy liver glycogen breakdown is initiated; it’s so powerful that glucose levels in organs and blood can increase to 40-60 times above normal. The thing is, this is a double-edged sword: if glucose needs to be maintained as a pool of cryoprotectant to prevent intracellular freezing, it (for the most part) can’t be used to feed glycolysis and maintain energy levels. Sure, with most metabolic processes shut off as a result of being an ice block ice, the energy demand isn’t terribly high. Still, the levels do need to be kept up to maintain cell viability, as a “life-support” system, as well as for the energetic strain associated with thawing. So where does the needed energy come from?
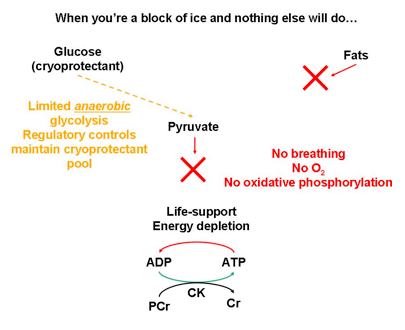
Fig. 3. In freeze-tolerant organisms, lack of respiration and lack of (frozen) blood circulation leads to ischemic hypoxia. Oxygen-dependent fatty acid oxidation and oxidative phosphorylation are not possible, leading to diminished ATP levels. CK can draw upon PCr reserves in certain tissues and organs to maintain energy levels.
In organs and tissues where phosphocreatine reserves are present, such as muscle, frozen frogs can draw upon them (Fig. 3) to maintain energy levels and the all-important life-support. In organs without phosphocreatine, different metabolic strategies come into play.
My focus, when it came to molecular mechanisms of wood frog adaptation, was reversible protein phosphorylation. Surely enough, given that CK is a much-needed enzyme in freeze-tolerance, its kinetic parameters- and enhanced ability to mobilize phosphocreatine energy reserves- are regulated by phosphorylation. As with any other enzyme or pathway regulated by post-translational modification, only certain protein kinases will do the job. I tried phosphorylating CK in vitro by stimulating several major metabolic kinases, but only calcium/calmodulin-dependent protein kinase (Ca2+/CaMK) and adenosine monophosphate-dependent protein kinase (AMPK) had a detectable kinetic effect (Fig. 4).
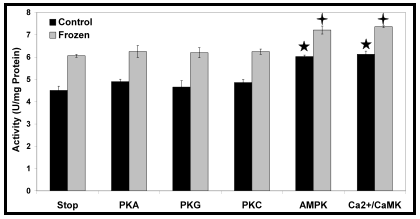
Fig. 4. In Rana sylvatica, several protein kinases were stimulated in vitro in an attempt to phosphorylate CK. However, out of those tested, only two- including the AMP-activated protein kinase (AMPK)- had a detectable effect on CK activity. Replotted from Dieni and Storey, 2009.
Why even bother to mention that AMPK regulates CK activity? I’ve given you enough to chew on, haven’t I? Well, I told you this article was about the surprising roles that an enzyme can sometimes play, and I recently discovered one for CK that surprised even me.
.
.
.
.
Creatine kinase and its role in… insulin secretion and anti-diabetic drugs?
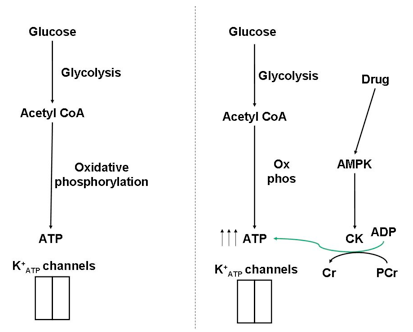
Fig. 5. The import of glucose, and its catabolism in pancreatic β-cells results in an increase in ATP levels, closing K+ATP channels and stimulating insulin secretion. However, this ATP increase may only be transient, resulting in a short insulin release- particularly within diabetics. Anti-diabetic drugs stimulate AMPK, and CK in turn. The localization of CK proximal to K+ATP channels shields the channels against changes in bulk nucleotide concentrations and enhances the insulin release.
First off, as much as I’d like to provide all the necessary background behind the molecular mechanisms of insulin secretion and anti-diabetic drugs (metformin and others), there’s only so much I can put into a BenchFly article. If you’re not very familiar with these mechanisms I’d suggest starting off here for insulin secretion and here for anti-diabetic drugs.
I recently read a paper (Düfer et al., 2010) that describes how anti-diabetic drugs enhance insulin secretion by activating AMPK. But wait… in normal physiology, glucose-stimulated insulin secretion works by catabolising glucose in β-cells, increasing ATP levels. With an increase in ATP, AMP levels would be low and AMPK wouldn’t be active. But by stimulating AMPK via anti-diabetic drugs, AMPK targets can be active in spite of the high glucose levels (and high ATP levels) that accompany insulin secretion. These AMPK targets, of course, include CK. In a more active state, CK can therefore maintain high ATP levels in the microenvironment of K+ATP channels, and enhance insulin secretion beyond the glucose stimulus alone (Fig. 5).
When we think of enzymes, we often think of them in a very narrow role that may only represent a small fraction of their physiological potential. What moments ago may have seemed to you like an enzyme that works only within the first few seconds of jogging or bench-pressing, actually keeps an organism alive during the months it spends frozen, and works with anti-diabetic drugs to enhance insulin secretion. If you poke your head around metabolism, you’ll see that creatine kinase is hardly unique in this respect.
What enzymes have you come across that have roles outside of the textbook norm?
.
.
References
- Dieni, C.A. and Storey, K.B. (2009) Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B 152: 405-412.
- Düfer, M., Noack, K., Krippeit-Drews, P. et al. (2010) Activation of the AMP-activated protein kinase enhances glucose-stimulated insulin secretion in mouse β-cells. Islets 2: 156-163.
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
.
.


alan@benchfly
wrote on October 7, 2011 at 1:15 pm
Cyclooxygenase-2 (COX-2) is another interesting example. For years, arachidonic acid was thought to be the sole substrate for COX-2, but about a decade ago it was shown that COX2 also oxidizes endocannabinoids, which opened up an entirely new biological role for the enzyme. Interestingly, it turns out that weak competitive active-site inhibitors of arachidonic acid oxygenation are actually potent inhibitors of endocannabinoid oxygenation – even though the inhibitor targets the same substrate binding site on the enzyme.
So in this case, not only was the enzyme found to have unexpected biological roles, but so too do the inhibitors.
The mechanism of substrate-selective inhibition was just discovered:
http://www.nature.com/nchembio/journal/vaop/ncurr…